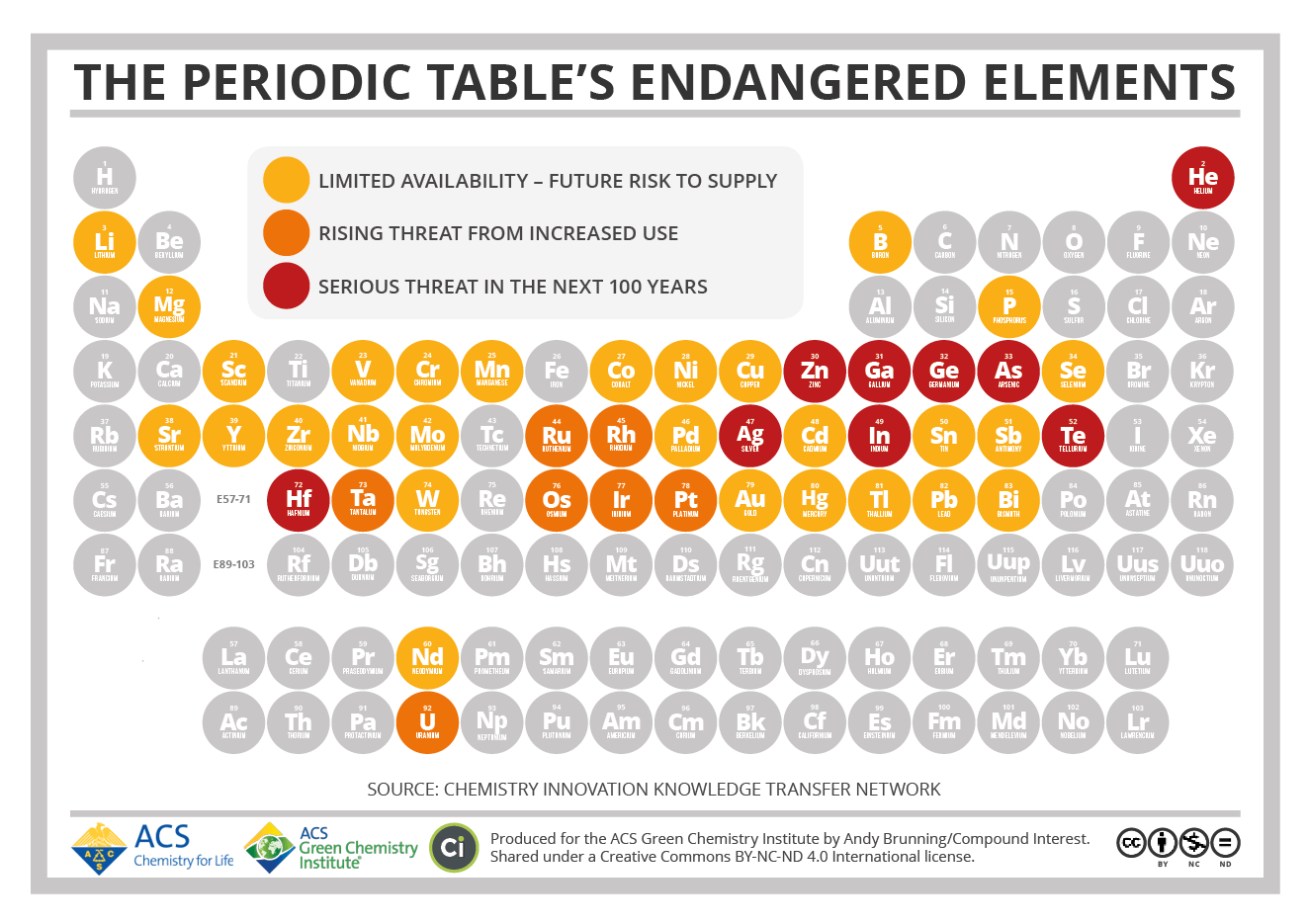
Source: Compound Interest
The Periodic Table’s Endangered Elements
August 26, 2015 8:00pm by Barry Ritholtz
This content, which contains security-related opinions and/or information, is provided for informational purposes only and should not be relied upon in any manner as professional advice, or an endorsement of any practices, products or services. There can be no guarantees or assurances that the views expressed here will be applicable for any particular facts or circumstances, and should not be relied upon in any manner. You should consult your own advisers as to legal, business, tax, and other related matters concerning any investment. The commentary in this “post” (including any related blog, podcasts, videos, and social media) reflects the personal opinions, viewpoints, and analyses of the Ritholtz Wealth Management employees providing such comments, and should not be regarded the views of Ritholtz Wealth Management LLC. or its respective affiliates or as a description of advisory services provided by Ritholtz Wealth Management or performance returns of any Ritholtz Wealth Management Investments client. References to any securities or digital assets, or performance data, are for illustrative purposes only and do not constitute an investment recommendation or offer to provide investment advisory services. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Past performance is not indicative of future results. The content speaks only as of the date indicated. Any projections, estimates, forecasts, targets, prospects, and/or opinions expressed in these materials are subject to change without notice and may differ or be contrary to opinions expressed by others. The Compound Media, Inc., an affiliate of Ritholtz Wealth Management, receives payment from various entities for advertisements in affiliated podcasts, blogs and emails. Inclusion of such advertisements does not constitute or imply endorsement, sponsorship or recommendation thereof, or any affiliation therewith, by the Content Creator or by Ritholtz Wealth Management or any of its employees. Investments in securities involve the risk of loss. For additional advertisement disclaimers see here: https://www.ritholtzwealth.com/advertising-disclaimers Please see disclosures here: https://ritholtzwealth.com/blog-disclosures/
What's been said:
Discussions found on the web:Posted Under
Previous Post
China's Market and Policy Timeline

Elements are neither created nor destroyed, save in a nuclear reactor or the core of a star, hence they cannot be endangered. Commercially viable mines may run out, but that is different from say an endangered species that once gone, is gone forever. Elements are still in existence but finely spread.
Holiday bus loads of Mexicans and Asians make them in US maternary wards.
That’s true of most elements, but not for helium as it escapes earth’s atmosphere and disappears into space. Once we run through what we have, we’re pretty much out (save any we can produce in hypothetical future fusion reactors).
Send robot miners to gas giant planets with sufficiently high gravity to hang onto their Helium, collect and refine it, and send it back to Mother Earth for kids’ balloons and voice tricks …
Jeff Bezos is probably working on a scheme to do this right now, so that Amazon can put helium instead of air into those cushioning bag inserts, so as to make packages lighter for their drones to deliver.
And as for Phosphorous, there are cemeteries all over the globe that can be mined to extract the phosphorous buried within.
Actually, it gets collected by politicians which is why they start squeaking out odd sounding noises during primary season. Recycling politicians may help delay the shortage.
I used to worry about Helium running out, but then I found out there’s actually quite a bit of it in the crust (second most common element in the universe after all and constantly being produced by natural radioactive decay). It’s currently a by-product of fracking for example that could be captured if it were worthwhile.
Also, fusion reactors already produce Helium from Hydrogen. They just don’t produce a net energy gain.
For a lot of these, as others have said, it’s a matter of ‘at what cost?’, but I would particularly worry about some of the heavier elements like Cadmium, Induim, and Tellurium that are used in modern electronics and are also pretty rare.
Lets take copper as an example: Today we are mining ores that were waste 100 years ago (In fact sometime we run leach extraction on old mine dumps). So the more important question is to state at what cost. As an other example the waters that spewed out of the mine in Co contain some of the metals that are said to be in potential short supply. Today its not economical to extract them but tommorrow? Or another waste water example. consider the Berkeley pit in Butte Mt, (an old open pit mine connected to the underground workings around Butte.) It is economical to extract the copper from that water at least in some quantity.
In addition for metals at least very high recycling rates are feasible. (indeed some thieves recycle copper from unabandoned buildings )
Or take Iron ore where the concentration of todays ores is far lower than the first ores out of the Mesabi range, and again most iron can be recycled, both at the fab plant where scraps have been recycled for years, and from cars and appliances over time.
Landfill mining is a very possible source for many of these down the road.
Right now many of the “recycled” electronics are just being dumped on the ground in places like China. A real recycling program down the road would recycle many of the metals used in modern electronics. Since many of the rare earths etc. are being mined in places like China, manufactured there, and then transported and sold here, we should actually be accumulating more of them. However, modern economic theory doesn’t recognize this as a value and simply re-exports them back to China.
The recycling process is already underway: http://www.greenbiz.com/article/apple-microsoft-motorola-wring-new-revenue-out-e-waste
BTW – re: Hg
There is lots of mercury out there – it is just in very low concentrations. Learning how to concentrate it from surface water and sediments would provide an almost endless source. You would be astonished at how many tons of mercury some marshes hold from industrial discharges as well as atmospheric deposition from coal-fired power plants. Also, just trapping the mercury before release from the stacks at the coal-fired power plants would probably provide much of the future supply – that would be a win-win as it would provide a source without opening new mines while reducing contamination of surface water and fish.
Silver is endangered? From what, hoarding?